Background: Mixed phenotype acute leukemia is a rare disease characterized by an expanded blast population exhibiting multiple lineage features. It is a diagnosis of exclusion, and we and others have observed that AML-MRC or therapy-related AML cases may also exhibit similar features. We therefore sought to determine whether multi-lineage expression is present in de-novo and therapy-related MDS and whether it has an impact on clinical outcomes.
Methods: We reviewed pathology, flow cytometry, cytogenetic, and molecular reports from patients seen at Memorial Sloan Kettering Cancer Center between 1996 and 2020 and identified 472 patients diagnosed with MDS using a combination of custom natural language processing tools and manual review. Cox proportional hazards modeling was performed to assess the contribution of patient characteristics, pathology, flow cytometric, cytogenetic, and molecular characteristics to overall survival (OS). Fisher's exact testing was used to assess the association of individual features.
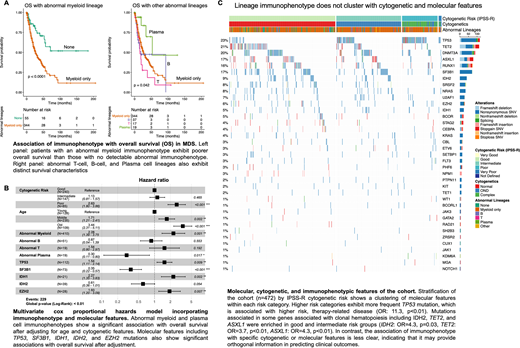
Results: We found that an abnormal myeloid lineage signature was associated with poorer OS after adjusting for age, cytogenetics, and molecular features (HR=2.3, p<0.01). In contrast, an abnormal plasma cell signature was associated with better OS (HR=0.3, p<0.01) (Figure A). Other abnormal lineages did not contribute significantly to OS although there were relatively few cases with abnormal T-cell markers (n=19). Mutations in TP53, IDH1, and EZH2 were associated with poorer OS (TP53: HR=1.5, p<0.01; IDH1: HR=2.3, p<0.01, EZH2: HR=1.9, p<0.01) and mutations in SF3B1 and IDH2 were associated with better OS (SF3B1: HR=0.35, p<0.01; IDH2: HR=0.6, p=0.05) (Figure B). TP53 mutation was enriched among patients with intermediate and poor risk cytogenetic groups (OR=11.3, p<0.01), consistent with higher risk therapy-related disease. IDH2, TET2, and ASXL1, which have been associated with clonal hematopoiesis, were enriched in the good and intermediate risk cytogenetic groups (IDH2: OR=4.3; p=0.03, TET2: OR=3.7, p<0.01; ASXL1: OR=4.3, p<0.01), although only IDH2 had a significant association with OS. Interestingly, there was a significant association of plasma cell lineage abnormalities with SRSF2 mutation (OR=3.5, p=0.05), but SRSF2 mutation was not an independent contributor to the global survival model (Figure C).
Conclusion: Routine flow cytometric and molecular assessment of diagnostic bone marrows can provide added prognostic value in MDS. Identification of an abnormal myeloid lineage is associated with higher risk disease, while identification of abnormal plasma cell features is associated with lower risk disease. SRSF2 mutation is also associated with abnormal plasma cell features, but does not contribute independently to OS. Abnormal T-cell features may also provide prognostic value, although assessment in a larger cohort will be necessary. We confirm previous findings indicating that TP53 mutation is associated with higher risk disease while SF3B1 mutation is associated with better outcomes characteristic of MDS-RARS. In addition, our data suggests that IDH1 and EZH2 are associated with poorer outcomes and that IDH2 is associated with lower risk disease.
Tallman:Bioline rx: Membership on an entity's Board of Directors or advisory committees; Delta Fly Pharma: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Abbvie: Research Funding; Cellerant: Research Funding; Orsenix: Research Funding; ADC Therapeutics: Research Funding; BioSight: Membership on an entity's Board of Directors or advisory committees, Research Funding; Glycomimetics: Research Funding; Rafael: Research Funding; Oncolyze: Membership on an entity's Board of Directors or advisory committees; UpToDate: Patents & Royalties; Novartis: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; KAHR: Membership on an entity's Board of Directors or advisory committees; Rigel: Membership on an entity's Board of Directors or advisory committees. Levine:Morphosys: Consultancy; Astellas: Consultancy; Janssen: Consultancy; Lilly: Consultancy, Honoraria; Isoplexis: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; C4 Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Imago: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Loxo: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Qiagen: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Prelude Therapeutics: Research Funding; Gilead: Honoraria; Novartis: Consultancy; Amgen: Honoraria; Roche: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Xiao:Stemline Therapeutics: Research Funding. Glass:Gerson Lehman Group: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.